Background: Patients with hematologic malignancies and/or hematopoietic stem cell transplantation (HSCT) recipients often require platelet transfusion support for extended periods. This can result in platelet transfusion refractoriness (PTR) from HLA alloimmunization in up to 30% of individuals. These medically complex patients may also have splenomegaly, fevers and other factors which contribute to PTR. Consequently, they may demonstrate variability in immediate platelet recovery (at 0-2 hours post-transfusion), and in subsequent platelet consumption (at 18-24 hours) despite receiving HLA-selected platelet products. We sought to examine patient and product related factors which impacted platelet transfusion response in a cohort of HLA-alloimmunized platelet transfusion refractory patients.
Methods: From April 2021 to June 2023, HLA-alloimmunized (PRA >50%) subjects who required three or more HLA selected platelet units were identified at the Fred Hutchinson Cancer Center/University of Washington. Data were obtained on patient demographics, diagnoses, Panel Reactive Antibodies (PRA %) and factors shown to be associated with poor platelet responses (spleen size, disseminated intravascular coagulation (DIC), bleeding, selected medications, history of hematopoietic stem cell transplant (HSCT) and/or graft versus host disease (GVHD), ABO compatibility, and product age). Platelet corrected count increments (CCI) were calculated for each transfusion. Non-HLA factors potentially contributing to CCIs at 0-2 and 18-24 hours post-transfusion were compared among HLA-compatible (either 4/4 HLA matched without HLA antigen mismatches, or with permissive mismatches (defined as cumulative a mean fluorescent intensity (MFI) <10,000 on the Luminex donor specific HLA antibody testing platform)) and HLA incompatible transfusions (cumulative MFI >10,000 or random donor apheresis products).
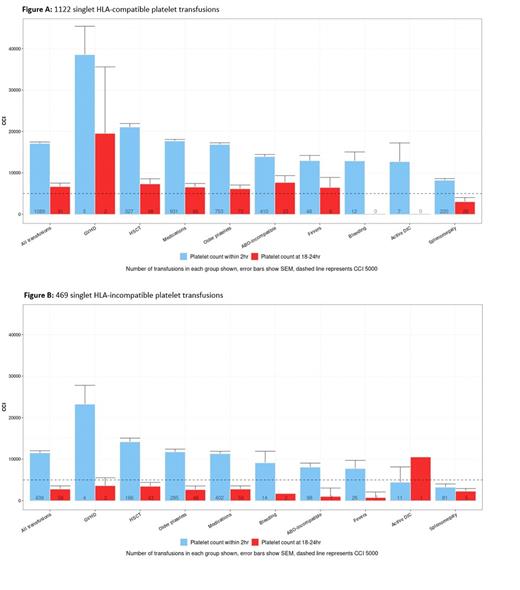
Results: Of the 420 patients screened, 75 had sufficient records for inclusion. These subjects received 2729 platelet transfusions, an average of 36 transfusions per patient over 5.2 (± 4.1) months. Post transfusion platelet counts were available for 1528 transfusions. All platelet products were leukoreduced, irradiated, single donor apheresis products. Patients were predominantly Caucasian (70.7%; n=53/75), female (82.7%; n=62/75), aged 57 (±14.9) years. The primary diagnosis was Acute myelogenous leukemia or myeloproliferative disorder in most cases (88%; n=66/75). 58.7% (n=44/75) of patients were HSCT recipients (3 auto-; 41 allo-HSCT). Average PRA among patients was 72.4%±22.2%. 25 patients had splenomegaly. Among HLA-compatible transfusions, average CCIs at 0-2 and 18-24 hours were 17,088 and 6,666. In HLA-mismatched/random donor transfusions, average CCIs at 0-2 and 18-24 hours were 11,489 and 2,786; HLA compatible products increased CCIs by 5,599 and 3880 at 0-2 and 18-24 hours. In both groups splenomegaly had the most consistent association with poor CCI at both time intervals (Figures A and B).
Conclusions: In this cohort of individuals with HLA-alloimmunization mediated PTR, the administration of HLA-compatible products offset the impact of other detrimental factors generating adequate CCIs at 0-2 and 18-24 hours. Among patients with splenomegaly, CCIs were lower despite HLA compatible product administration. In the same individuals, HLA-incompatible/random donor products negatively impacted CCIs overall and the negative effect of other concomitant non-immune/product-related factors was additive.Our data highlights the need to account for non-HLA related conditions while evaluating CCI response following each HLA-selected product transfusion, and to further explore any interactions among such factors. Next steps include conducting a multivariate analysis and developing a predictive model for platelet transfusion response in HLA-alloimmunized recipients who have concomitant non-immune or product related risk factors.
Disclosures
Panch:Sobi: Consultancy, Speakers Bureau; Sanofi: Consultancy, Other: Advisory Board. Tsang:Roche: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal